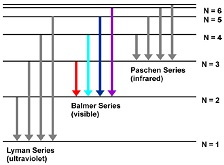
Spectral lines of Hydrogen atom
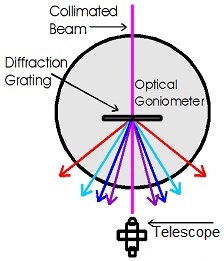
Schematic of spectral dispersion
Note: stainless steel optical table or breadboard not provided
Features
Stable aluminum rail for easy alignment
Hydrogen-Deuterium lamp
Detailed instructional manual
Introduction
The Balmer series is referred to as a set of discrete emission spectral lines of the Hydrogen atom. The visible Balmer series involves the electron transitions from the higher energy levels (n ≥ 3) to the second energy level (n = 2) where n is the principal quantum number of the electron.
The wavelengths of the Balmer series of the Hydrogen atom can be calculated from equation 1/λ = R (1/22-1/n2), n=3, 4, 5, ..., where R is an empirical constant called the Rydberg constant (R=1.096776x107 m-1).
This apparatus uses a diffraction grating to disperse the collimated beam of a Hydrogen-Deuterium lamp and a digital protractor stage along with a telescope to measure the diffraction angles of the Balmer series lines. Once the wavelengths are determined, the experimental value of the Rydberg constant can be derived.
Experiments and Applications:
1. Measurement of the Balmer Series: The primary experiment allows students to observe the visible lines of the Balmer series in hydrogen and measure the diffraction angles using the diffraction grating and telescope.
2. Determination of Wavelengths: The diffraction angles measured during the experiment are used to calculate the corresponding wavelengths of the spectral lines.
3. Calculation of the Rydberg Constant: Using the measured wavelengths and applying the Balmer series equation, students can derive the experimental value of the Rydberg constant.
4. Understanding the Hydrogen Spectrum: Students will understand how the transitions between electron energy levels in the hydrogen atom produce specific spectral lines, which can be analyzed to gain insights into atomic structure.
The instruction manual contains comprehensive materials including experimental configurations, principles and step-by-step instructions. Please click to view some sample pages of the instruction manual.
Specifications
| Item | Specifications |
| Hydrogen-Deuterium Lamp | Wavelengths: 410, 434, 486, 656 nm |
| Digital Protractor | Resolution: 0.1° |
| Condensing Lens | f = 50 mm |
| Collimating Lens | f = 100 mm |
| Transmissive Grating | 600 lines/mm |
| Telescope | Magnification: 8 x; diameter of objective lens: 21 mm with internal reference line |
| Optical Rail | Length: 74 cm; aluminum |
Part List
| Description | Qty |
| Optical rail | 1 |
| Carrier | 3 |
| X-translation carrier | 1 |
| Optical rotation stage with digital protractor | 1 |
| Telescope | 1 |
| Lens holder | 2 |
| Lens | 2 |
| Grating | 1 |
| Adjustable slit | 1 |
| Telescope holder (tilt adjustable) | 1 |
| Hydrogen-Deuterium lamp with power supply | 1 set |

Spectral lines of Hydrogen atom

Schematic of spectral dispersion